Our Science
NEXT GENERATION OF PRECISION
RNAi THERAPIES
Based on the scientific discovery of Switch’s co-founders, the company’s proprietary CASi platform combines advantageous properties of both single and double-stranded RNAs in a single molecule, allowing for efficient self-delivery and uptake, potency and durable gene knockdown, as well as cell selectivity. Switch is leveraging the unique properties of CASi molecules to develop the next generation of precision RNAi therapies for central nervous system (CNS) diseases.
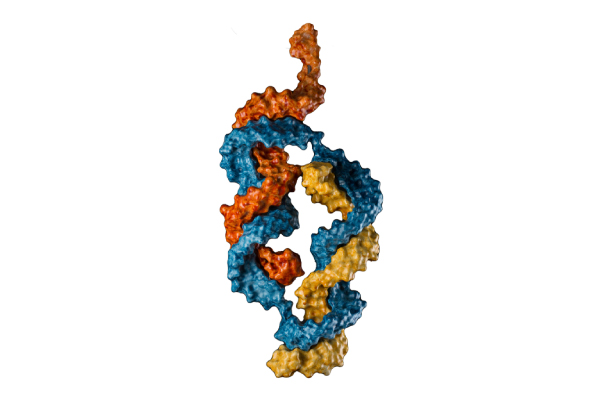
CASi: A NOVEL CLASS OF DRUGS
Switch Therapeutics has developed a novel class of drugs called Conditionally Activated siRNAs (CASi). Each CASi molecule employs a small interfering RNA (siRNA) structure with added extensions to dock to a RNA-based molecular “switch”. The addition of this sensor gives CASi two, novel properties:
Efficient self-delivery and uptake, potency and sustained duration of gene knockdown.
The combination of a unique mechanism for effective self-delivery as well as cell selectivity, enables new avenues for next generation RNAi therapy.
The unique ability to target RNAi activity to specific cell populations with unique expression of sensor genes. By enabling cell selective knockdown of targets by RNAi, CASi has the ability to improve the therapeutic window for existing targets and open the door for numerous, therapeutic applications.
The Platform
COMBINING ADVANTAGEOUS PROPERTIES OF SINGLE AND DOUBLE-STRANDED RNAs IN A SINGLE MOLECULE
Modern oligonucleotide medicines for gene knockdown belong to two different categories; short single-stranded molecules called antisense oligonucleotides (ASOs) and short, double-stranded molecules called siRNAs. These current gene knockdown modalities have both incredible promise and known limitations. There is no current gene knockdown modality that is highly potent, exhibits good distribution and has cell-selective capabilities.

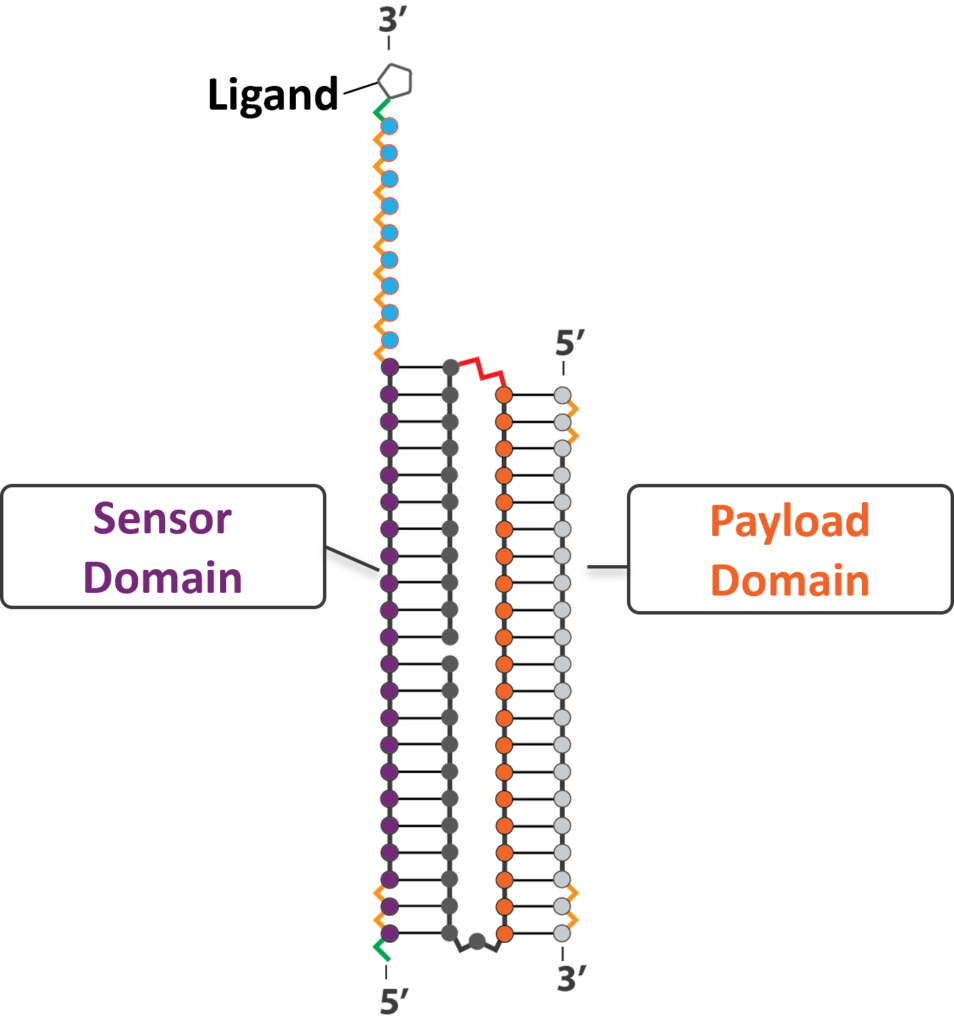
CASi MOLECULES COMBINE THE BEST OF BOTH APPROACHES
On the CASi molecule, the single stranded toehold domain in the sensor gives CASi efficient self-delivery properties. Once in the cell, the sensor can decouple from the siRNA, allowing use of RNAi for efficient gene knockdown. This best-of-both-worlds approach helps substantially improve delivery and activity. As a result, CASi has broad therapeutic applicability with the potential to deliver the next generation of precision RNAi therapies for CNS indications.
OUR PIPELINE
We believe our CASi platform has broad applicability, with the potential to treat a range of central nervous system diseases, as well as systemic indications.
Internally, our efforts are initially focused on advancing multiple programs for the treatment of neurodegenerative diseases. With these programs, we leverage our ability to achieve high CNS potency, long duration and deep brain distribution and are advancing towards IND-enabling studies in the near-term.
We continue to advance programs that leverage our differentiated cell–selective RNAi activity.
We also intend to explore opportunities to expand our CASi platform into new areas through potential pharmaceutical and biotechnology collaborations.
